Biostatistics and data science
Clinical statistics
Staburo offers pharmaceutical/biotech companies, contract research organisations, and medical device manufacturers the opportunity to use our statistical expertise.
Our company is a biostatistics and data science service provider with expertise in the field of clinical research. The expertise ranges from the design of clinical phase I-IV trials (statistical input to study protocols, writing of statistical analysis plans, randomization, sample size calculation) and data analysis with SAS and R. For the calculation of pharmacokinetic and pharmacodynamic parameters Winnonlin is used. High quality standards are ensured by independent validation (e.g. independent review, code review or double programming). Our statistics experts supported numerous clients in submissions to FDA and EMA.
We have solid experience in oncology (RECIST) and complex study designs (e.g. adaptive, Bayesian BLRM, outcome depending switching/crossover, biomarker) and the implementation of complex methods in macros (RPSFT, IPE, IPCW). We have set up a simulation toolbox in SAS to find the optimal design and to verify the performance of methods and macros. Our toolbox is also useful for developing custom-fit translational research trials and analyses strategies.
Staburo supports pharmaceutical companies with data preparation for public disclosure on clinicaltrials.gov, EudraCT and other registers.
For the analyses, CDISC standards with ADaM datasets are used to ensure traceability, reproducibility, and compatibility across studies. If necessary, we consult on the anonymisation of clinical data.
Our team members are up-to-date on the EU data transparency legislation, because we participated in public disclosure initiatives for different clients.
We support biosimilars development programs, which play a crucial role in delivering biological medicines to a huge amount of patients. Depending on the client’s needs, we also work on-site for requested periods.

Translational medicine & biomarkers
- Development of protocols and statistical analysis plans
- Analysis of high-throughput biomarker data
- Identification and validation of biomarkers using complex statistical models
- Application of innovative study designs
- Statistical support for assay validation
Statistical programming with CDISC
- Analysis datasets
- Tables, listings and figures
- CDISC (SDTM and ADaM) mapping
- Program validation
- Biomarker identification
- Implementation of crossover correction methods in SAS

You need biostatistics or statistical programming support?
Pharmacokinetics/-dynamics
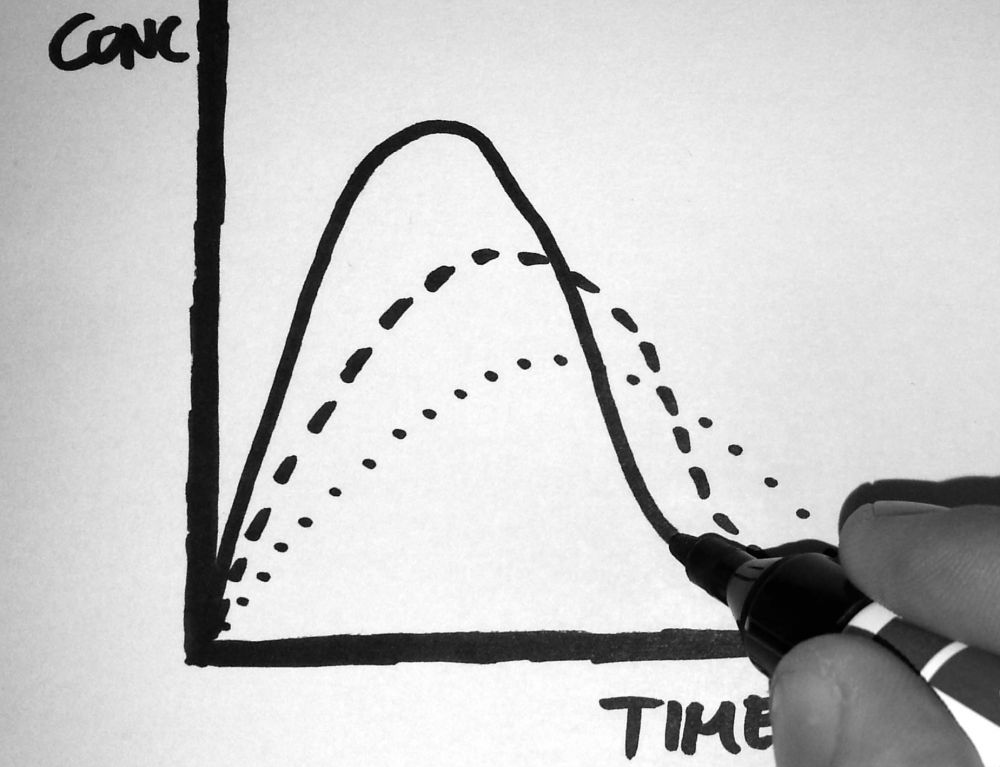
- Noncompartmental pharmacokinetics analyses and compartmental pharmacokinetics/simulations,
- Ascending dose
- Repeat dose
- Bioavailability and bioequivalence
- Drug interaction studies
- Pharmacodynamic and pharmacokinetic/pharmacodynamic modelling and BLRMs
Health technology assessment

Non-clinical statistics

- Statistical and analysis planning for medical device trials
- Design of Experiments statistical input and recommendations
- Stability testing
- Dissolution testing
- JoS
- FDS
- Interaction with authorities (e.g. FDA, EMA) focusing on statistical design or data analyses
Contact us today!
Data transparency
Disclosure with Staburo

Transparency made easy.
Staburo is happy to fulfil your transparency goals whilst ensuring compliance with all relevant disclosure rules, regulations, and requirements. Our experienced disclosure experts will guide you through the entire disclosure process, from protocol registration to the posting of results on public registries like ClinicalTrials.gov, EudraCT, CTIS or HMA-EMA catalogue of RWD studies (former ENCePP). We also support your RegAffairs team in the preparation of EU clinical trial application part I data.
Protocol review for disclosure readiness
Disclosure smooth right from the start.
Drafting protocols with disclosure in mind smooths the disclosure process and helps avoid potential compliance issues. To ensure your protocol is tailored for disclosure, our team of experts will support and guide your trial team during protocol drafting, finishing with a protocol review from a disclosure perspective that will ensure all relevant requirements have been addressed.

Protocol registration and study record maintenance
Timely registration matters.
Registration demands juggling timelines, maintaining study records, updating amendments and study dates. Let us simplify your agenda and do this time intensive job for you. Our disclosure managers are experienced in handling trial registration in a timely fashion and in spite of difficulties, aligned with our clients’ transparency policies and in compliance with the applicable registries’ regulations. We are pleased to maintain and regularly update your study records with respect to trial timeline changes, protocol amendments and company policies.
Results posting
Successful results posting is crucial.
The posting of results presents evidence-based medical knowledge to the world and is the final important step in what is typically a long and substantial program of research. Since this step is so critical, we at Staburo are determined to post your results successfully regardless of the complexity of the trial design. Our experienced team will guarantee the posting of results, in full compliance with the relevant registries once your trial has been completed.

Our data transparency experience

Use our experience for your disclosure needs!
We have successfully handled protocol registration and results posting of 400+ trials, ranging from standard Phase I to complex pivotal trials, as well as real-world evidence generated from non-interventional studies. We will, of course, take care of any quality issues arising from reviews by the registries.
Our disclosure experts are dedicated to meet your needs! We are looking forward to working with you.
Data sharing and data redaction
Your trial has been successfully completed and now some data sharing requests are coming in. You need help in assessing such requests either internally or with external stakeholders? Or you need help getting the data into shape in the form of document redaction for publication according to Policy 0070, ClinicalTrials.gov or journal specifications, or even the anonymization of individual patient data? Staburo will support you here.
You will benefit from broad experience, garnered from the review of close to 2,000 documents and counting.
Let’s have a chat!

Contact us today to learn more about our support in data transparency!
Bioinformatics
Our bioinformatics experience

Staburo’s bioinformatics consultancy services include state-of-the-art bioinformatics data analysis and bioinformatical programming. We e.g. specify and review bioinformatics related analyses sections in statistical analysis plans (SAP) or biomarker research projects. Furthermore, we give input to and write data analysis sections of analytical plans, describing conducted analyses.
Application of existing workflows to analyse genomic data, as well as establishment of new workflows and analysis strategies to analyse genomics data, is one of our main expertise.
Our bioinformatics team produces program code, to generate analysis datasets and outputs, based on analysis plans, e.g. biomarker/statistical analysis plans. We create, communicate and send out final results to responsible statisticians, programmers or data managers. Due to our extensive knowledge from previous projects and sound academic education, we support our clients in the establishment, documentation and conduct of integrative analyses of “omics” data.
Validation of assays (e.g. microarrays), or also program code (i.e. to compare analysis datasets and to compare results with the results shown in outputs based on analysis plans) also forms a big part of our services, to help our clients providing adequate results within agreed delivery dates.
Contact us today for bioinformatics support!
Services
Expert tasks
Staburo services include advanced statistical support, such as:- Consulting on statistical issues
- Crossover correction in oncology (IPCW, IPTW, IPE, RPSFT methods in SAS macros)
- Statistical models for high-dimensional genomic data
- In-depth QoL analyses
- Multiple imputation
- Presentations and statistics workshops
- Data transparency (ClinicalTrials.gov, EudraCT)
- Bayesian Methods (e.g. BLRM for dose escalation, Safety evaluation)
- Adaptive designs
- Data simulation


Regulatory submission support
Staburo supports you with regulatory submissions, e.g.:
- Preparation of submission of dossier and other submission documents for FDA, EMA, and other authorities
- Clinical Overview (CO)
- Integrated Summaries of Effectiveness (ISE)
- Integrated Summaries of Safety (ISS)
- Investigator’s Brochure (IB)
- Summary of Product Characteristic (SmPC)
- Periodic Benefit Risk Evaluation Report (PBRER)
- Agency interactions
- Preparation/input to HTA appraisals (e.g. NICE and IQWiG)
- Statistical expert reports
Clinical trials
Staburo has a very strong focus on statistics for clinical trials and performs these specific tasks:- Clinical trial planning (phases I-IV)
- Trial and project statistician tasks
- Sample size calculation
- Randomisation (including emergency envelopes)
- Statistical programming with SDTM and ADaM (SAS, R)
- Validation (e.g. SAS: source code review, double programming)
- Interim analyses
- Survival analysis

Therapeutic areas
- Oncology – strong focus
- Respiratory – many projects as preferred provider
- Allergology
- Cardiovascular
- Central nervous system
- Dentistry
- Dermatology
- Gastroenterology
- Gynaecology
- Haematology
- Immunology
- Infectious diseases
- Metabolic
- Ophthalmology
- Pneumology
- Surgery
Looking for flexible data science solutions?
Memberships and awards


